Hepatitis B with Delta agent, also known as “superinfection,” is a serious viral disease that affects the liver. It is distinguished by a more rapid and severe course than hepatitis B virus. Patients infected with this virus are at risk of developing cirrhosis and liver cancer.
According to official statistics, approximately 20 million individuals worldwide are afflicted with this particular form of hepatitis. However, the risk of hepatitis D extends to an additional 325 million individuals worldwide, as the hepatitis D virus attaches itself to the already existing hepatitis B virus.
For an extended period, there was no effective therapy for hepatitis B+D, leaving patients with this dangerous disease without hope. Nevertheless, collaborative scientific research conducted by German and Russian scientists led to a significant advancement: the development of a substance, Bulevirtide, which can obstruct the virus from entering cells and impede the replication of hepatitis D.
The stages of scientific research and the release of a unique drug are discussed.
The initial phase of Bulevirtide research commenced in 2012 and concluded in 2016. During this period, German and Russian scientists collaborated to identify an efficacious approach to combating hepatitis B and D. The objective of the research was to develop a substance that could block the virus from entering cells and limit its replication. Preclinical laboratory studies were conducted to assess the safety and efficacy of the novel substance.
In 2018, the US Food and Drug Administration (FDA) granted the substance Bulevirtide the status of “breakthrough therapy.” This was a significant event, as such a status is only awarded to drugs that represent a fundamentally new treatment for severe or life-threatening diseases.
A year after this event, in 2019, the first drug with Bulevirtide received a registration certificate in Russia under the commercial name Mirkludex B. The much-anticipated therapy was finally made available to patients with hepatitis B and the Delta agent.
In 2020, the European Medicines Agency (EMA) recommended approval of Bulevirtide for sale in the European Union, but under a different commercial name, Hepcludex. This represented a significant advance in the global effort to combat hepatitis B and Delta agent infections, with implications for millions of patients in Europe.
The objective of this study is to determine the efficacy of Bulevirtide in the treatment of hepatitis B and D.
Bulevirtide is a synthetic analog of a small fragment (47 amino acids) of the outer envelope of the hepatitis B virus (HBV). The hepatitis B virus employs a specific protein on its surface, designated pre-S1, to establish a connection with liver cells via their NTCP protein. This process enables the virus to gain access to the cells.
The drug is created in a manner analogous to that of the viral protein, and it can also bind to NTCP. However, in contrast to the aforementioned protein, it does not facilitate the virus’s access to the cells; rather, it impedes this process. This process prevents the virus from replicating and spreading, thereby protecting the liver from further inflammation and complications.
By impeding the invasion of viruses into cells, Bulevirtide facilitates the eradication of these pathogens by the body’s immune system. The drug is also effective against hepatitis D, which cannot activate without hepatitis B. By blocking the entry of the B virus into cells, the drug simultaneously limits the multiplication of hepatitis D and reduces its impact on the body, reducing the symptoms and effects of the disease. Consequently, the reduction of inflammation facilitates the deceleration of the development of fibrosis and cirrhosis of the liver.
What distinguishes Myrcludex B from Hepcludex in the treatment of hepatitis B and D?
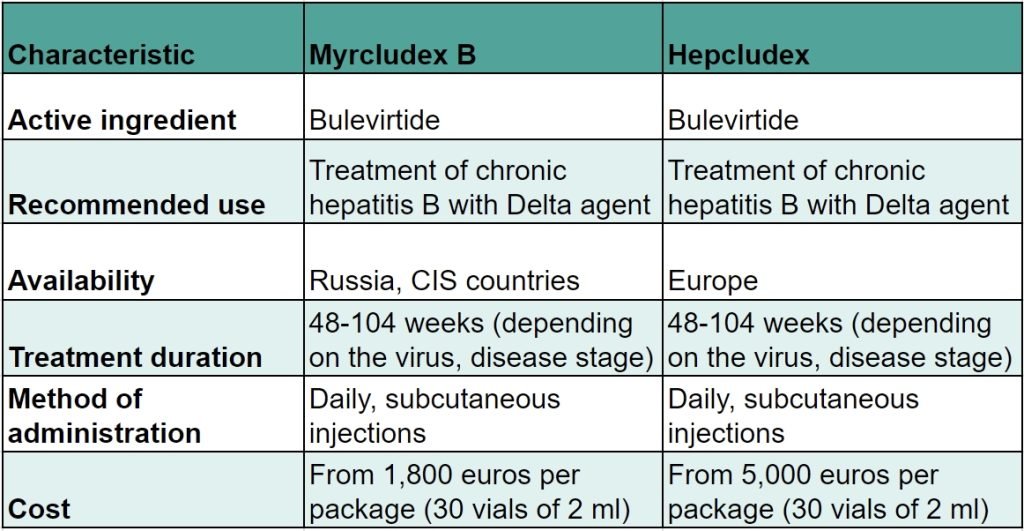
Upon examination of the data pertaining to the stages of the trials, it becomes evident that Mirkludex B and Hepcludex are, in fact, the same drug, differing only in their respective trade names.
In Russia and the CIS countries, the drug is referred to as Myrcludex B.
In Europe, the drug is referred to as Hepcludex.
The active ingredient, manufacturer, dosage, and recommendations for use are identical for these drugs. The distinction lies in the nomenclature, with the availability of the drugs varying by country. It is also noteworthy that the cost of pharmaceuticals can vary considerably, despite the identical active ingredient and raw materials. This discrepancy is frequently attributed to the distinctive requirements and costs associated with obtaining registration certificates for various countries.
The pharmaceutical industry is well-acquainted with the practice of naming drugs differently for different geographical markets. This phenomenon is known as “rebranding,” which can be attributed to a multitude of factors, including registration restrictions and patent rights.
It is a matter of interest to ascertain whether Myrkludex B can be employed in place of Hepcludex.
The analysis of the properties of Myrcludex B and Hepcludex, as well as reports reflecting the experience of using these medicines for viral hepatitis B and D, indicate that it is possible to use one drug instead of the other.
Both drugs contain the active ingredient Bulevirtide and act in a similar manner by blocking the NTCP receptor on liver cells, thereby preventing hepatitis B and D viruses from entering the cells. The principal dissimilarities between these pharmaceuticals are their respective costs and availability in disparate countries. Nevertheless, a considerable number of patients residing in Europe, the United States, and other foreign countries are able to procure pharmaceuticals from abroad for their treatment.
A comparison of the cost of a single pack of Hepcludex and Mirkludex B reveals a significant discrepancy, particularly when considering the duration of the treatment course.
The average difference in the cost of purchasing one package is 3,350 euros. If we consider that the patient requires approximately 12 packages for the course of treatment, then the savings achieved by purchasing a course of Myrkludex B instead of Hepcludex are considerable, amounting to 40,200 euros over the course of the annual treatment.

